Stem Cells Tourism
Stem
Cells
Tourism
Over the last few decades, a new type of tourism has emerged worldwide. It is stem cell tourism, a global phenomenon that has surged in popularity, particularly in the Arab world, in which people travel across geographical borders to receive treatments that are not available in their home country, at clinics or facilities that specialize in stem cell therapy. Patients seek this type of treatment in the hope of curing diseases for which conventional medicine has failed to find a definitive cure, such as paralysis, or finding more effective ways to treat diseases such as arthritis, while others place high hopes in cosmetic stem cell treatments such as skin rejuvenation and anti-aging (1) based on dazzling advertisements that spread across social media and videos posted by influencers, despite the fact that these treatments are largely experimental.
While medical tourism has numerous benefits, promoting common legitimate therapies proven to be safe and effective around the world, many clinics are marketing stem cell therapies that are deemed unproven procedures by scientists, making them equally accessible to tourists promoted to enhance tourism and profit from the last ray of hope desperate patients have (1).
These clinics and centers are not subjected to appropriate inspection; hence, not only do they lack organized mechanisms (2), but they also lack transparency in their treatment protocols. The cells and blood products used are often of unknown source, raising concerns about their quality and type, including what claims to use stem cells while, in fact, they use platelet-rich plasma treatments. As for others, they may claim to use stem cells extracted from donated umbilical cord blood (4), from people whose health history has not been verified, and these cells have not been tested for diseases, posing the risk of disease transmission, such as AIDS (acquired immunodeficiency syndrome) (5,6). They are clinics that do not disclose potential hazards and do not provide follow-up service to ensure patient safety (7). Furthermore, little is known about long-term treatment safety without documentation of treated cases, protocols used, and efficacies.
Although few complications of stem cell tourism were reported, they do not accurately reflect the exact scale of these complications. A team of researchers from the University of Connecticut and the Ohio State Neuroscience Research Institute in the United States conducted a survey assessing neurologists’ experiences with stem cell tourism outcomes to determine the prevalence of complications associated with unapproved stem cell therapies. Of the 204 neurologists who responded, one in four reported that at least one patient developed complications following unapproved stem cell therapy; such as acute cardiomyopathy, liver and skin injuries (4), meningitis (8), tumors (9, 10), nerve damage, and even death (11,12,13), in addition to the enormous financial losses.

It is of no doubt that Stem cell research has demonstrated great success in pre-clinical studies (14), showing promising treatment results in numerous disorders, such as diabetes, heart disease, arthritis, Alzheimer’s disease, paralysis, multiple sclerosis, macular degeneration (15), aging diseases, and hair loss (16), however, clinical trials are still ongoing to provide additional evidence of efficacy and safety before they can be approved for use internationally (17) and thus have not been subjected to adequate clinical safety tests. On the contrary, they may pose health risks (18). Hence, these clinics market illegal treatments as proven, not even in the context of clinical trials, but as certain, effective, and safe treatments through their websites and advertisements on social media, and through testimonies from those who have had this type of treatment. Unfortunately, this advertising is misleading because it contradicts scientific research recommendations (1).
Stem cell transplantation is a complex procedure that should only be performed under strict medical supervision and has been internationally approved for the treatment of limited disorders, including leukemia, some Immune and metabolic illnesses, as well some tumors such as lymphomas and multiple myelomas (15,19). However, it has not yet been approved safe to treat any other diseases.
The approval process for medical treatments is lengthy and complex. Therapeutic procedures or medications are only licensed after extensive testing over several years of studies and clinical trials. This includes stem cell treatments, which cannot be approved until they have passed all necessary tests demonstrating efficacy and safety, as well as documentation of all possible side effects to ensure patient safety. These studies begin with laboratory experiments and progress through several stages of clinical trials; the first phase is the safety and feasibility phase conducted on a small group of patients to evaluate the safety and side effects. The second and third phases are conducted on a larger group of patients to determine the treatment’s effectiveness and safety; then compare it to other available treatments. After treatment approval is obtained, the fourth and final phase of clinical trials begins to gather more information on treatment effectiveness and long-term safety (1, 17, 20, 21).
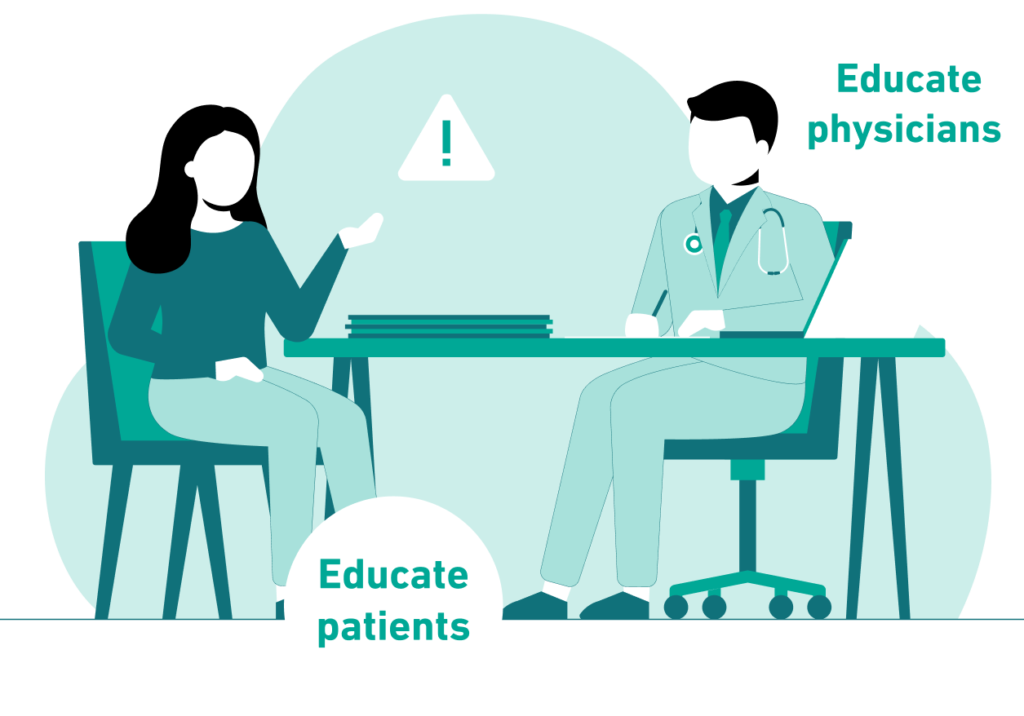
It is worth noting the importance of raising public awareness about stem cell therapy to correct misinformation. This can be done by developing simplified scientific content highlighting approved stem cell treatments, unproven interventions, and the role of scientific research in distinguishing between experimental treatments for legitimate research and fraudulent therapies. Setting up clear communication channels to consult experienced, highly trained professionals can greatly educate patients and provide the advice and guidance they need regarding approved and unapproved stem cell therapies. On the other hand, unapproved treatments must be prevented by tightening regulations, enforcing medical malpractice laws, supervising direct health marketing and implementing rules against misleading online or social media marketing. In addition, creating a national platform for reporting any complication from these types of unproven treatments can significantly help the medical community collect data to determine the extent of the problem.
Additionally, it is necessary to educate physicians about effective discussion techniques for constructive interaction with patients. This can help provide advice and guidance regarding stem cell tourism, warn and explain the risks, benefits, and treatment alternatives, communicate basic facts to them to convince them, or even refer them to competent organizations that can answer their questions and provide additional information. For patients seeking information on the validity and effectiveness of a particular treatment, several platforms are available such as the International Society for Stem Cell Research (ISSCR) (22), which publishes stem cell research findings and provides guidance and information on promising and proven treatments. On a local level, the Saudi Society of Blood and Marrow Transplantation (SSBMT) (23) may provide information in its field.
References:
- Fung, M., Yuan, Y., Atkins, H., Shi, Q., Bubela, T. Responsible translation of stem cell research: An assessment of clinical trial registration and publications. Stem Cell Reports 2017; 8(5):1190–1201.
- Finch, S. (2014). Thailand top destination for medical tourists. CMAJ: Canadian Medical Association Journal 2014;186(1): E1–E2.
- Crooks, V. A., Snyder, J. (2011). Medical tourism: What Canadian family physicians need to know. Canadian Family Physician 2011; 57(5):527–529.
- Bauer G, Elsallab M, Abou-El-Enein M. Concise review: a comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Transl Med. 2018;7(9):676–85.
- Halme D G, Kessler D A, FDA regulation of stem-cell-based therapies. N. Engl. J. Med. 2006;355:1730-1735.
- Turner L. US stem cell clinics, patient safety, and the FDA. Trends in Molecular Medicine 2015;21(5):271-3
- Murdoch B, Zarzeczny A, Caulfield T. Exploiting science? A systematic analysis of complementary and alternative medicine clinic websites’ marketing of stem cell therapies. BMJ Open. 2018;8(2):e019414
- Dobkin B H, Curt A, Guest J. Case report. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair 2006;20(1):5-13
- Thirabanjasak D, Tantiwongse K, Thorner PS. Case report. Angiomyeloproliferative lesions following autologous stem cell therapy . J Am Soc Nephrol. 2010;21(7):1218-22.
- Amariglio N, Hirshberg A, Scheithauer B W, Gideon Rechavi. Donor-Derived Brain Tumor Following Neural Stem Cell Transplantation in an Ataxia Telangiectasia Patient. PLoS Med 2009;6:e1000029.
- Julian K, Yuhasz N, Rai W, Salerno J, Imitola J. Complications from “Stem Cell Tourism” in Neurology. Ann neurol 2020;88:661–668
- Večerić-Haler Ž, Borštnar Š, Luzar B et al. Multiorgan failure with fatal outcome after stem cell tourism. Eur J Med Res 2021;26(5).
- Gallagher G, Forrest DL . Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer 2007;109:84–92
- Rao M, Mason C, Solomon S. Cell therapy worldwide: an incipient revolution. Regen Med. 2015;10(2):181–91.
- https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/Miscellaneous/Pages/StemCells.aspx
- McArdle A, Senarath-Yapa K, Walmsley G G, Hu M, et al. The role of stem cells in aesthetic surgery: Fact or fiction? Plastic and Reconstructive Surgery 2014;134(2):193–200.
- Lindvall O, Hyun I. Medical innovation versus stem cell tourism. Science 2009; 324(5935):664–1665.
- https://stemcellnetwork.ca
- Master Z, Resnik, D B. Stem-cell tourism and scientific responsibility.Stem-cell researchers are in a unique position to curb the problem of stem-cell tourism. EMBO Reports 2011;12(10),992–995.
- Mummery C, Van de Stolpe A, Bernard A J, Roelen B A, Clevers H, Stem Cell Tourism, Stem Cells 2nd ed. 2014 ;11:291-314
- https://saudihematology.org.sa/ar
- www.isscr.org




